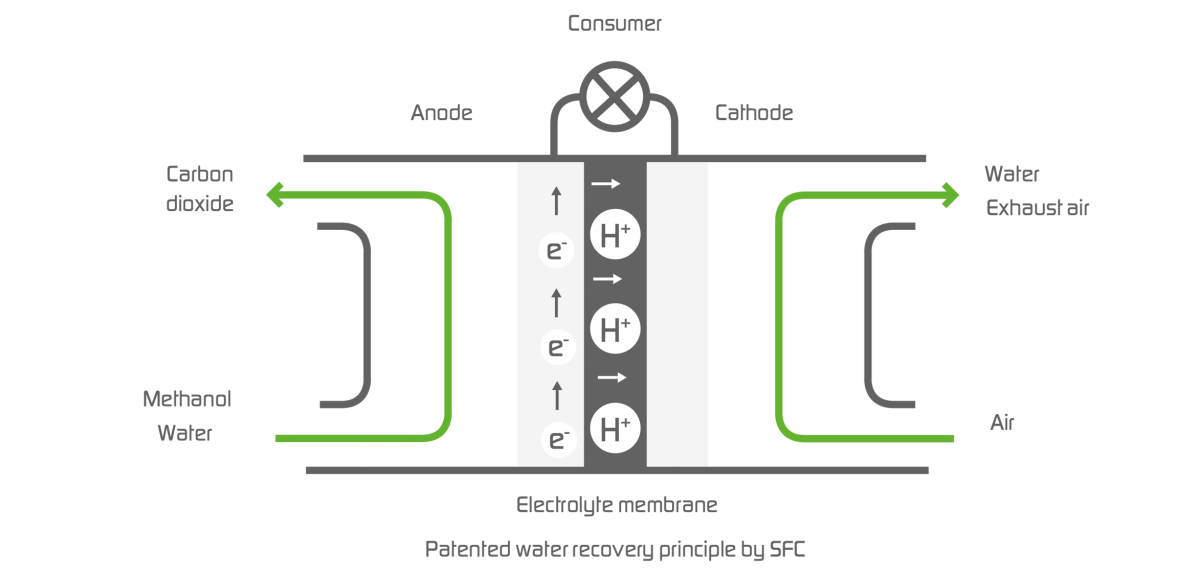
A fuel cell is a galvanic cell that converts the chemical reaction energy of a continuously supplied fuel and oxidant into electrical energy. The underlying principle is an electrochemical process that is also called “cold combustion”. In a way, it’s the reverse of electrolysis. A fuel cell does not store energy but is a converter that generates power. The energy for power production is supplied by the fuel.